Cellular Respiration
Cellular respiration is the release of energy from organic compounds by metabolic
chemical oxidation in the mitochondria within each cell. Cellular respiration involves a series of
enzyme- mediated reactions.
The equation below shows the complete oxidation of glucose. Oxygen is required for this
energy releasing process to occur.
C6H1206 + 602 CO2 + 6H20 + 686 kilocalories of energy/mole of glucose oxidized
By studying the equation above, you will notice there are three ways cellular respiration
could be measured. One could measure the:
1. Consumption of 02 (How many moles of 02 are consumed in cellular respiration?)
2. Production of CO2. (How many moles of CO2 are produced in cellular respiration?)
3. Release of energy during cellular respiration.
In this experiment, the relative volume of 02 consumed by germinating and nongerminating
(dry) peas at two different temperatures will be measured.
Background Information
A number of physical laws relating to gases are important to the understanding of how the
apparatus that you will use in this exercise works. The laws are summarized in the general gas law
that states:
PV = nRT
where
P is the pressure of the gas,
V is the volume of the gas,
n is the number of molecules of gas,
R is the gas constant (its value is fixed), and T is the temperature of the gas (in o K).
This law implies the following important concepts about gases:
1. If temperature and pressure are kept constant, then the volume of the gas is directly proportional
to the number of molecules of the gas.
2. If the temperature and volume remain constant, then the pressure of the gas changes in direct
proportion to the number of molecules of gas present.
3. If the number of gas molecules and the temperature remain constant, then the pressure is
inversely proportional to the volume.
4. If the temperature changes and the number of gas molecules is kept constant, then either
pressure or volume (or both) will change in direct proportion to the temperature.
It is also important to remember that gases and fluids flow from regions of high pressure to regions
of low pressure.
In this experiment, the CO2 produced during cellular respiration will be removed by
potassium hydroxide (KOH) and will form solid potassium carbonate (K2CO3) according to the
following reaction:
CO2+ 2KOH K2CO3+ H20
Since the CO2 is being removed, the change in the volume of gas in the respirometer will
be directly related to the amount of oxygen consumed.
In the experimental apparatus (Figures 5.1 and 5.2), if water temperature and volume
remain constant, the water will move toward the region of lower pressure. During respiration,
oxygen will be consumed. Its volume will be reduced, because the CO2 produced is being
converted to a solid. The net result is a decrease in gas volume within the tube, and a related
decrease in pressure in the tube. The vial with glass beads alone will permit detection of any
changes in volume due to atmospheric pressure changes or temperature changes.
The amount of O2 consumed will be measured over a period of time. Six respirometers
should be set up as follows:
| Respirometer |
Temperature |
Contents |
| 1 |
Room |
Germinating Seeds |
| 2 |
Room |
Dry Seeds + Beads |
| 3 |
Room |
Beads |
| 4 |
10oC |
Germinating Seeds |
| 5 |
10oC |
Dry Seeds + Beads |
| 6 |
10oC |
Beads |
1. Both a room temperature bath (approximately 25oC) and a 10oC bath should be set up
immediately to allow for time to adjust the temperature of each. Add ice to attain 10oC
2. Respirometer 1: Obtain a 100 mL graduated cylinder and fill it with 50 mL of H20. Drop in
25 germinating peas and determine the amount of water that was displaced (which is equivalent to
the volume of peas). Record the volume of 25 germinating peas. Remove these peas and place
them on a paper towel. They will be used in respirometer 1.
3. Respirometer 2: Refill the graduated cylinder with 50 mL of H20. Drop 25 dried peas (not
germinating) into the graduated cylinder and then add enough beads to attain a volume equivalent
to that of the expanded germinating peas. Remove these peas and beads and place them on a
paper towel. They will be used in respirometer 2.
4. Respirometer 3: Refill the graduated cylinder with 50 mL of H20. Determine how many
beads would be required to attain a volume equivalent to that of the germinating peas. Remove
these beads and place them on a paper towel. They will be used in respirometer 3.
5. Repeat the procedures above to prepare a second set of germinating peas, dry peas plus beads,
and beads for use in respirometers 4, 5, and 6, respectively.
6. To assemble the six respirometers, obtain six vials, each with an attached stopper and pipette.
Place a small wad of absorbent cotton in the bottom of each vial and, using a dropper, saturate the
cotton with 15% KOH. Make sure that the respirometer vials are dry on the inside. Do not get
KOH on the sides of the respirometer. Place a small wad of dry cotton on top of the KOH soaked
absorbent cotton (Figure 5.1). It is important that the amounts of cotton and KOH be the
same for each respirometer.
Figure 1: Assembled Respirometers
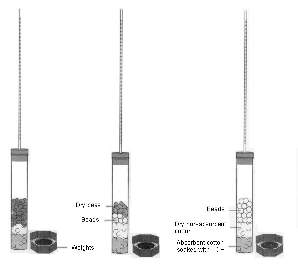 7. Place the first set of
germinating peas, dry peas + beads, and beads in vials 1, 2, and 3, respectively.
Place the second set of germinating peas, dry peas plus beads, and beads in vials 4, 5, and 6,
respectively. Insert the stopper fitted with the calibrated pipette. Place a
weighted collar on each end of the vial (Figure 5.2).
7. Place the first set of
germinating peas, dry peas + beads, and beads in vials 1, 2, and 3, respectively.
Place the second set of germinating peas, dry peas plus beads, and beads in vials 4, 5, and 6,
respectively. Insert the stopper fitted with the calibrated pipette. Place a
weighted collar on each end of the vial (Figure 5.2).
8. Make a sling of masking tape attached to each side of each of the water baths to
hold the pipettes out of the water during an equilibration period of seven minutes. Vials 1, 2, and 3
should rest in the room temperature water bath
(approximately 25oC) and vials 4, 5, and 6 should rest in the 10oC water bath (Figure 5.2).
Figure 2: Respirometers in the Water Bath

9. After the equilibration period of seven minutes, immerse all six
respirometers entirely in their water baths. Water will enter the pipettes
for a short distance and then stop. If the water continues to move into a
pipette, cheek for leaks in the respirometer. Work swiftly and
arrange the pipettes so that they can be read through the water at the
beginning of the experiment. They should not be shifted during the
experiment. Hands should be kept out of the water bath after the experiment has started. Make
sure that a constant temperature is maintained.
10. Allow the respirometers to equilibrate for three more minutes and then record, to the nearest
0.01 mL, the initial position of water in each pipette (time 0). Cheek the temperature in both baths
and record in Table 1. Every 5 minutes for 20 minutes, take readings of the water's position in
each pipette, and record the data in Table 1.
Table 1: Measurement of 02 Consumption by Soaked and Dry Pea Seeds at Room
Temperature (25oC) and 10oC Using Volumetric Methods
Temp
(oC) |
Time
(min) |
Beads Alone |
Germinating Peas |
Dry Peas and Beads |
| Reading
at time X |
Diff.* |
Reading
at time X |
Diff.* |
Corrected
diff.** |
Reading at
time X |
Diff.* |
Corrected
diff.** |
|
Initial - 0 |
|
|
|
|
|
|
|
|
| 0 - 5 |
|
|
|
|
|
|
|
|
| 5 - 10 |
|
|
|
|
|
|
|
|
| 10 - 15 |
|
|
|
|
|
|
|
|
| 15 - 20 |
|
|
|
|
|
|
|
|
|
Initial - 0 |
|
|
|
|
|
|
|
|
| 0 - 5 |
|
|
|
|
|
|
|
|
| 5 - 10 |
|
|
|
|
|
|
|
|
| 10 - 15 |
|
|
|
|
|
|
|
|
| 15 - 20 |
|
|
|
|
|
|
|
|
* Difference = (initial reading at time 0) - (reading at time X)
** Corrected difference = (initial pea seed reading at time 0 - pea seed reading at time X) - (initial bead reading at
time 0 - bead reading at time X)
11. Graph the results from the corrected difference column for the germinating peas and the dry
peas at both temperatures.
Questions
1. This experiment uses a number of controls. Identify at least three of the controls, and describe
the purpose of each.
2. Describe and explain the relationship between the amount of O2 consumed and time.
3. From the slope of the four lines on the graph, determine the rate of O2 consumption (in mL O2
/min) of germinating and dry peas during the experiments at both temperatures.
4. Why is it necessary to correct the readings from the peas with the readings from the beads?
5. Explain the effect of germination (versus nongermination) on pea seed respiration.
6. What is the purpose of KOH in this experiment?
7. Why did the vial have to be completely sealed around the stopper?
8. If you used the same experimental design to compare the rates of respiration of a 25 g mammal
and a 25 g reptile, at 10oC, what results would you expect? Explain.
9. If respiration in a small mammal were studied at both 21oC and 10oC, what results would you
expect? Explain.
10. Explain why water moved in the respirometers.
 7. Place the first set of
germinating peas, dry peas + beads, and beads in vials 1, 2, and 3, respectively.
Place the second set of germinating peas, dry peas plus beads, and beads in vials 4, 5, and 6,
respectively. Insert the stopper fitted with the calibrated pipette. Place a
weighted collar on each end of the vial (Figure 5.2).
7. Place the first set of
germinating peas, dry peas + beads, and beads in vials 1, 2, and 3, respectively.
Place the second set of germinating peas, dry peas plus beads, and beads in vials 4, 5, and 6,
respectively. Insert the stopper fitted with the calibrated pipette. Place a
weighted collar on each end of the vial (Figure 5.2).
