

PLANT PIGMENTS AND PHOTOSYNTHESIS
EXERCISE 4A: Plant Pigment Chromotography
Paper chromatography is a useful technique for separating and identifying pigments and other molecules from cell extracts that contain a complex mixture of molecules. The solvent moves up the paper by capillary action, which occurs as a result of the attraction of solvent molecules to the paper and the attraction of solvent molecules to one another. As the solvent moves up the paper, it carries along any substances dissolved in it. The pigments are carried along at different rates because they are not equally soluble in the solvent and because they are attracted, to different degrees, to the fibers in the paper through the formation of intermolecular bonds, such as hydrogen bonds.
Beta carotene, the most abundant carotene in plants, is carried along near the solvent front because it is very soluble in the solvent being used and because it forms no hydrogen bonds with cellulose. Another pigment, xanthophyll, differs from carotene in that it contains oxygen. Xanthophyll is found further from the solvent front because it is less soluble in the solvent and has been slowed down by hydrogen bonding to the cellulose. chlorophylls contain oxygen and nitrogen and are bound more tightly to the paper than are the other pigments.
Chlorophyll a is the primary photosynthetic pigment in plants. A molecule of chlorophyll a is located at the reaction center of photosystems. Other chlorophyll a molecules, chlorophyll b, and the carotenoids (that is, carotenes and xanthophylls) capture light energy and transfer it to the chlorophyll a at the reaction center. Carotenoids also protect the photosynthetic system from the damaging effects of ultraviolet light.
Procedure
1. Obtain a 50 ml graduated cylinder which has 1 cm of solvent in the bottom. This cylinder is tightly stoppered because this solvent is volatile, and you should be careful to keep the stopper on as much as possible.
2. Refer to Figure 1 to construct the apparatus. Cut a piece of chromatography paper which will be long enough to reach the solvent. Cut one end of this filter paper into a point. Draw a pencil line 1.5 cm above the point.
3. Use a quarter to extract the pigments from the chosen plant leaf cells. Place a small section of leaf on the top of the pencil line. Use the ribbed edge of the quarter to crush the cells. Be sure that the pigment line is on top of the pencil line. You should repeat this procedure 8 to 10 times, being sure to use a new portion of the leaf each time.
4. Place the chromatography paper in the cylinder so that the pointed end is barely immersed in the solvent. Do not allow the pigment to be in the solvent.
5. Stopper the cylinder. When the solvent is about 1 cm from the top of the paper, remove the paper and immediately mark the location of the solvent front before it evaporates.
6. Mark the bottom of each pigment band. Measure the distance each pigment migrated from the bottom of the pigment origin to the bottom of the separated pigment band. Record the distance that each front, including the solvent front, moved in Table 1. Depending on the species of plant used, you may be able to observe 4 or 5 pigment bands.
 |
 |
Table 1: Distance moved by Pigment bands (mm)
| Band Number | Band Distance (mm) | Band Color |
The relationship of the distance moved by a pigment to the distance moved by the solvent is a constant (for a given solvent) called Rf. It can be calculated for each pigment in a mixture using the formula:
Record the Rf values in Table 2
Table 2: Rf Values for all pigments
| Rf | Pigment |
| carotene (yellow to yellow-orange) | |
| xanthopyll (yellow) | |
| chlorophyll a (bright green to blue green) | |
| chlorophyll b (yellow green to olive green) | |
EXERCISE 4B: Photosynthesis: The Light Reaction
Light is a part of a continuum of radiation or energy waves. Shorter wavelengths of energy have greater amounts of energy. For example, high-energy ultraviolet rays can harm living tissues. Wavelengths of light within the visible part of the light spectrum power photosynthesis.
When light is absorbed by leaf pigments, electrons within each photosystem are boosted to a higher energy level and this energy is used to produce ATP and to reduce NADP to NADPH. ATP and NADPH are then used to incorporate C02 into organic molecules, a process called carbon fixation.>
Design of the Exercise
Photosynthesis may be studied in a number of ways. For this experiment, a dye-reduction technique will be used. The dye-reduction experiment tests the hypothesis that light and chloroplasts are required for the light reactions to occur. In place of the electron acceptor, NADP the compound DPIP (2,6-dichlorophenol-indophenol), will be substituted. When light strikes the chloroplasts, electrons boosted to high energy levels will reduce DPIP. It will change from blue to colorless.
In this experiment, chloroplasts are extracted from leaves and incubated with DPIP in the presence of light. As the DPIP is reduced and becomes colorless, the resultant increase in light transmittance is measured over a period of time using a spectrophotometer. The experimental design matrix is presented in Table 3.
Table 3 Cuvettes
| 1
Blank |
2
Unboiled Chloroplasts, Dark |
3
Unboiled Chloroplasts, Light |
4
Boiled Chloroplasts, Dark |
5
No Chloroplasts | |
| Phosphate Buffer | 1 mL | 1 mL | 1 mL | 1 mL | 1 mL |
| Distilled H2O | 4 mL | 3 mL | 3 mL | 3 mL | 3 mL + 3 drops |
| DPIP | - | 1 mL | 1 mL | 1 mL | 1 mL |
| Unboiled Chloroplasts | 3 drops | 3 drops | 3 drops | - | - |
| Boiled Chloroplasts | - | - | - | 3 drops | - |
Procedure
1. Turn on the spectrophotometer to warm up the instrument and set the wavelength to 605 nm.
2. Refer to Figure 2 to set up an incubation area that includes a light, water flask, and test tube rack. The water in the flask acts as a heat sink by absorbing most of the light's infrared radiation while having little effect on the light's visible radiation.
Figure 2
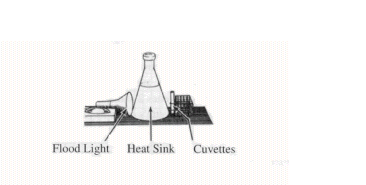
3. Be sure to keep these on ice at all times.
4. At the top rim, label the cuvettes 1, 2, 3, 4, and 5, respectively. Using lens tissue, wipe the outside walls of each cuvette (REMEMBER: handle cuvettes only near the top). Cover the walls and bottom of cuvette 2 with foil and make a foil cap cover for the top. Light should not be permitted inside cuvette 2 because it is a control for this experiment.
5. Refer to Table 2 to prepare each cuvette. Do not add unboiled or boiled chloroplasts yet.
6. Bring the spectrophotometer to zero by adjusting the amplifier control knob until the meter reads 0% transmittance. Cover the top of cuvette 1 with Parafilm® and invert to mix. Insert cuvette 1 into the sample holder and adjust the instrument to 100% transmittance by adjusting the light-control knob. Cuvette 1 is the blank to be used to recalibrate the instrument between readings. In other words, you will measure the light transmitted through the tube as a percentage of the light transmitted through this tube. For each reading, make sure that the cuvettes are inserted into the sample holder so that they face the same way as in the previous reading.
7. Obtain the unboiled chloroplast suspension, stir to mix, and transfer three drops to cuvette 2. Immediately cover and mix cuvette 2. Then remove it from the foil sleeve and insert it into the spectrophotometer's sample holder, read the % transmittance, and record it as the time 0 reading in Table 3. Replace cuvette 2 in the foil sleeve, and place it in the incubation test-tube rack. Turn on the flood light. Take and record additional readings at 5, 10, and 15 minutes. Mix the cuvette's contents just prior to each reading. Remember to use cuvette 1 occasionally to check and adjust the spectrophotometer to 100% transmittance.
8. Obtain the unboiled chloroplast suspension, mix, and transfer three drops to cuvette 3. Immediately cover and mix cuvette 3. Insert into the sample holder, read the % transmittance, and record in Table 4. Place cuvette 3 in the incubation test-tube rack next to cuvette 2. Take and record additional readings at 5, 10, and 15 minutes. Mix the cuvette's contents just prior to each reading. Remember to use cuvette 1 occasionally to check and adjust the spectrophotometer to 100% transmittance.
9. Obtain the boiled chloroplast suspension, mix, and transfer three drops to cuvette 4. Immediately cover and mix cuvette 4. Insert into the sample holder, read the % transmittance, and record in Table 4. Place cuvette 4 in the incubation test-tube rack next to cuvettes 2 and 3. Take and record additional readings at 5, 10, and 15 minutes. Mix the cuvette's contents just prior to each reading. Remember to use cuvette 1 occasionally to check and adjust the spectrophotometer to 100% transmittance.
10. Cover and mix the contents of cuvette 5. Insert it into the sample holder, read the % transmittance, and record in Table 4. Place cuvette S in the incubation test-tube rack next to tubes 2, 3, and 4. Take additional readings at 5, 10, and 15 minutes. Mix the cuvette's contents just prior to each reading. Remember to use cuvette 1 occasionally to check and adjust the spectrophotometer to 100% transmittance.
Table 4: Transmittance (%)
Time (min)
| Cuvette | 0 | 5 | 10 | 15 |
| 2 Unboiled/Dark | ||||
| 3 Unboiled/Light | ||||
| 4 Boiled/Light | ||||
| 5 No Chloroplasts |
11. Plot the percent transmittance versus time for the four cuvettes.
Questions
1. What factors are involved in the separation of the pigments?
2. Would you expect the Rf value of a pigment to be the same if a different solvent were used? Explain.
3. What is the purpose of DPIP in this experiment?
4. What molecule found in chloroplasts does DPIP "replace" in the experiment?
5. What is the source of the electrons that will reduce DPIP?
6. What is measured with the spectrophotometer in this experiment?
7. What is the effect of darkness on the reduction of DPIP? Explain.
8. What is the effect of boiling the chloroplasts on the subsequent reduction of DPIP? Explain.
9. What reasons can you give for the difference between percent transmittance between the live chloroplasts that were incubated in the light and those that were kept in the dark?