 9. Pour all the chlorophyll solution
into the large flat-sided glass container
on the teacher's bench. When several
groups have done this there will be
enough solution to demonstrate light
absorption by chlorophyll as described
in the next step.
9. Pour all the chlorophyll solution
into the large flat-sided glass container
on the teacher's bench. When several
groups have done this there will be
enough solution to demonstrate light
absorption by chlorophyll as described
in the next step.Properties and Composition of Chlorophyll
Materials
green leaves (preferably 5-10 geranium leaves and several spinach leaves)
alcohol
250 mL beaker
500 mL beaker
hot plate
crucible tongs
overhead projector
small flat-sided bottle
large flat-sided glass container
triangular glass prism
green food colouring
large test tube (20 x 150 mni)
chromatography paper
capillary tube
piece of wooden splint
aluminum foil
mortar and pestle
clean quartz sand
Procedure A Extraction of Chlorophyll from Leaves
1. Put about 300 mL of water in a 500 mL beaker. Bring the water to a boil.
2. Drop 5-10 geranium leaves into the boiling water. Remove them as soon as they become limp.
3. Transfer the leaves to a 250 mL beaker containing about 150 mL of boiling alcohol.
4. Boil the leaves in the alcohol for about 5 min. If the extraction does not appear complete, continue the boiling for a few more minutes.
5. Discard the leaves. Retain the green solution for Procedure B.
Procedure B Some Properties of Chlorophyll
6. Pour chlorophyll solution into the small flat-sided bottle until it is almost full.
7. Shine a bright light on one side of the bottle. (Use the projector.) View the solution from directly above by looking through the open neck of the bottle. Record your observations.
8. Repeat step 7 using the solution of green food colouring of about the same green colour as the chlorophyll solution. Record your observations.
 9. Pour all the chlorophyll solution
into the large flat-sided glass container
on the teacher's bench. When several
groups have done this there will be
enough solution to demonstrate light
absorption by chlorophyll as described
in the next step.
9. Pour all the chlorophyll solution
into the large flat-sided glass container
on the teacher's bench. When several
groups have done this there will be
enough solution to demonstrate light
absorption by chlorophyll as described
in the next step.
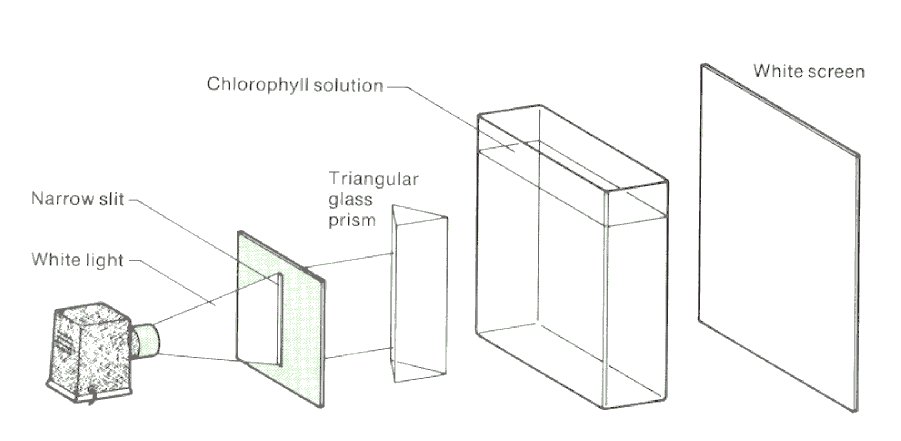
10. Set up the apparatus as shown in Fig. 1, but without the chlorophyll solution in place. Adjust the positions of the prism, projector, and screen until a complete colour spectrum appears on the screen. Note carefully the colours observed. Then insert the flat-sided container full of chlorophyll solution in the path of the beam of light as shown in Fig. 1. Note any changes that occur in the spectrum.
Procedure C Separation of the Pigments in Chlorophyll Extract
In this procedure you use a method called paper chromatography to separate the pigments that are in chlorophyll extract. The way this works is easily understood. You have seen water soaked up by a paper towel. Liquids appear to move well through some materials like paper and cloth. Suppose a substance is dissolved in the water moving up the paper towel. This substance will be carried up as the paper towel acts as a wick. How far it is carried up depends on several factors. One factor is the mass of the substance's particles. Another factor is the solubility of the substance in water.
Paper chromatography works much the same way. Chromatography paper replaces the paper towel and a solvent that will dissolve the substances to be separated replaces the water. In this investigation the substances to be separated are the pigments in the chlorophyll extract.
9. Collect a few green leaves.
10. Tear the leaves into pieces. Put the pieces in a mortar. Add about 5 mL of alcohol and a pinch of quartz sand. Grind the mixture until a deep green (almost black) solution is obtained. Add more leaves if the solution is not dark enough. This deep green solution is the chlorophyll extract.
11. Prepare a strip of chromatography paper about 1 cm wide and 20 cm long.
12. Using a pencil (not pen), draw a line across the filter paper about 2 cm above the tip of the paper. Use the capillary tube to spread one drop of the pigment extract along the line. Allow the paper to dry completely. Gentle fanning will help. Now spread a second drop of the pigment extract along the line. Allow it to dry. Keep repeating this until a dark green strip is present across the filter paper.
13. Place 3-4 mL of alcohol in the test tube.
14. Hang the filter paper in the test tube so that only the tip of the filter paper is in the alcohol. The strip of chlorophyll extract should be about 1 cm above the surface of the alcohol. Make sure that the filter paper hangs in the centre. It must not touch the wall of the test tube and must be hanging vertically.
15. Let the system stand until the solvent has almost reached the top of the paper. Record your observations immediately. Some of the colours change if they stand too long. In particular, note the number of bands of colour, the colour of each band, and the relative widths of the bands. You may wish to remove the paper from the test tube, dry it, and mark this information on the paper.
Questions
1. The chlorophyll extract that was obtained is green. The presence of a green pigment could create this colour. Is it possible that pigments with colours other than green are present? Explain. (the green could be a result of two or more pigments combined. Also, green could mask other lighter colors)
2. What was noted when the chlorophyll was observed from the top as the solution was being illuminated from the side? Explain this observation. (bright red. The pigment reflects some red light in that direction. In the cell, this light energy is used)
3. a) What effect did the chlorophyll extract have on the spectrum? (only green passed through)
b) What colours of the spectrum does chlorophyll absorb? (blue and red were absorbed)
c) What colours of the spectrum does chlorophyll transmit? (green absorbed)
4. a) What colours of the spectrum does this experiment suggest are most involved in photosynthesis? Why? (blue and red most involved because those colors were absorbed)
b) What colours of the spectrum does this experiment suggest are least involved in photosynthesis? Why? (green light least involved because it was transmitted)
c) Why does a geranium leaf appear green in daylight? (leaf absorbs little green light so it is reflected)
d) What colour would a geranium leaf would appear if it were illuminated only with red light? Why? (appears black because most red light is absorbed)
5. How many different bands of colour were formed? (four bands seen)
6. The yellow or yellow-orange pigments that travel highest on the paper are carotenes. The other yellow or yellow-green band (sometimes two bands) consists of xanthophylls. The dark green band is chlorophyll a. The lighter green (sometimes blue-green or even yellow-green) band is chlorophyll b. List the components of your chlorophyll extract in the order in which they appeared on the paper, from the top down. Calculate the Rf value for each pigment. Draw any conclusions you can from the relative widths and the distance they rose.
7. Why were all these colors not visible in the leaf? (chlorphylls are most abundant and darkest so they mask other pigments)